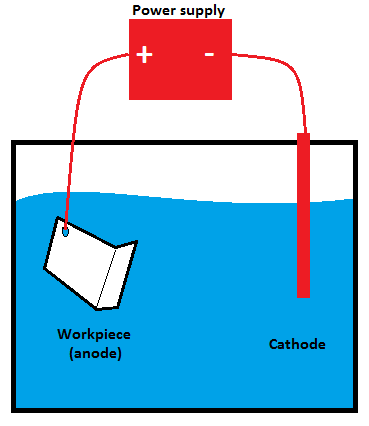
Anodizing is an electrolytic surface treatment most commonly used with aluminium components. It creates a hard, durable, corrosion-resistant, non-conductive, and often reflective oxide finish on the outside surface of the anodized part. It also makes the surface of the component easy to dye and paint due to the porous nature of the oxide layer. For this reason, it is often used as a pre-treatment for parts that will be dyed, coated or bonded.
Typical attributes of anodizing:
- Surface hardness, Vickers: 600-1000
- Coating thickness: .00002-.006” (0.05µm – 150µm)
- Processing temperature: 32-110ºF
Anodizing is accomplished with the workpiece submerged in a tank filled with an electrolytic acid solution. A current (typically low, from 5-20V) is passed through the solution via a cathode submerged in the solution, with the workpiece serving as the anode. Oxygen is released by the current at the surface of the aluminium, building up a layer of aluminium oxide on the outside of the part.
To prepare for anodizing, the part will normally be cleaned, chemically etched (or stripped), and rinsed. After anodizing, the part will be cleaned and rinsed again. Typically a plating shop will move the part through a series of tanks for each operation. This can be automated for large batches or done manually.
Typical Uses for Anodizing
- Preparing aesthetic consumer products for dyeing and painting. Some examples:
a) Personal electronics (smartphone/tablet cases)
b) Decorative colored accessories (carabiners, metal pens, flashlights)
c) Bicycle parts
- Adding corrosion resistance to aluminium airplane components.
- Protecting from chemical corrosion on parts exposed to fuels and other chemicals.
- Increasing scratch resistance.
- Preventing galling on threaded components.
Materials
Anodizing is most commonly used on aluminium. However, there are some applications where anodizing is used for other materials:
- Titanium: Normally anodized for use as jewelry, dental implants, and artwork (due to its tendency to form bright colors from anodizing).
- Zinc: Rarely anodized except in cases where a deep green color is desired. Forms a hard, wear-resistant oxide layer.
- Magnesium: Sometimes anodized to prime for painting. Requires additional sealing or treatment for corrosion resistance.
- Ferrous metals: Commonly anodized using nitric acid to form a hard, wear-resistant black oxide layer.
Iron and carbon steel: Not anodized, as iron oxides (rust) tends to corrode the material.
Mechanical design guidelines for anodizing
- Dimensional effects: When designing for anodizing, keep in mind that the surface will actually raise, as an oxide layer build up on the outside of the part. In tight-tolerance applications this additional layer will need to be accounted and dimensioned for. The surface will typically increase by ½ of the oxide layer thickness.
- Coverage limitations: Normally the part will not form an oxide layer where it is connected to the power source. So if it is hung by an aluminium wire through a hole, the surface of the hole will not anodize. So tooling must take this into account, or features must be built into the part to allow it to be held from a non-critical surface.
- Thermal stress cracking: Anodized layers are susceptible to thermal stress cracking at temperatures upward of 350K.
- Fatigue life effects: Anodizing can either increase or decrease the fatigue life of a part, either by reducing corrosion pitting or by propagating surface cracks. The effect will depend on the alloy and application, so make sure to refer to industry-specific literature.
- Sealing required: Due to the porous nature of oxide coatings, a sealing treatment is commonly used to reduce micro-cracks and prevent chemical corrosion.
Process variations
- Depending on the application, anodizing can be combined with other surface treatments such as chromate conversion coating (to increase electrical conductivity) or color dyeing.
- The two most common processes are Chromic Acid (type I) anodizing and Sulfuric Acid (type II and III) anodizing.
Type I is used for thin (.00002” to .00007”) coatings, typically to provide chemical resistance. Since chromic acid by itself does not attack Aluminium, post-anodizing cleaning and rinsing is not as critical as with other processes. - Type II, also known as “standard anodizing”, results in a thicker (.00007”-.0001”) layer and is used to prepare parts to be dyed and to increase wear and scratch resistance and lubricity.
- Type III, also known as “hard anodizing”, leaves an even thicker layer (.0005”-.006”) and is used to further increase wear and corrosion resistance, and thermal and electrical insulation. Thicker oxide layers require stricter process controls, often requiring refrigerated acid-baths and carefully timed voltage cycles.
- Nitric acid anodizing is used for ferrous metals, and results in a hard black oxide layer.
Tradenames / alternative names
- The term anodizing is used to cover a range of processes including hard anodizing, black oxide coating (for ferrous metals) and other specific variations of the process.
- Some anodizing processes are known by acronyms (for example, PAA for phosphoric acid anodizing) but these vary by industry.
Economics of the process
- It is possible to anodize parts with a set-up as simple as a bucket of acid and a battery. However, equipment costs are high, starting in the tens of thousands of dollars, for an industrial-scale line of tanks, controllers, chillers, and other requirements. However, tooling costs are very low and sometimes non-existent depending on the parts being treated.
- Alternative processes include simple painting, chromating and phosphating. Anodising is the most expensive but also the most effective of these processes.
Environmental implications
In general it is an environmentally friendly process, but some anodizing processes require aggressive chemicals which must be managed with a recycling loop and disposed of properly. The most common waste products, aluminium sulphate and aluminium hydroxide, can be recycled.
Advantages
- Low tooling costs
- Low labor and setup costs
- Large range of process variables and anodized layer thicknesses
- Compatible with other processes
Disadvantages
- High machinery costs
- Not useful for iron or carbon steel
- Often requires secondary treatment or sealing
- Expensive compared to alternative processes
References
- Machinery’s Handbook, 29th edition. Erik Oberg & Franklin Jones. Industrial Press, 2014.
- Materials & Design – The Art & Science of Material Selection in Product Design. Mike Ashby & Kara Johnson. Elsevier Ltd, 2010.
Aluminium Anodizers Council website: aluminium.com